“20min Biological Indicator for Steam” has been added to your cart. View cart
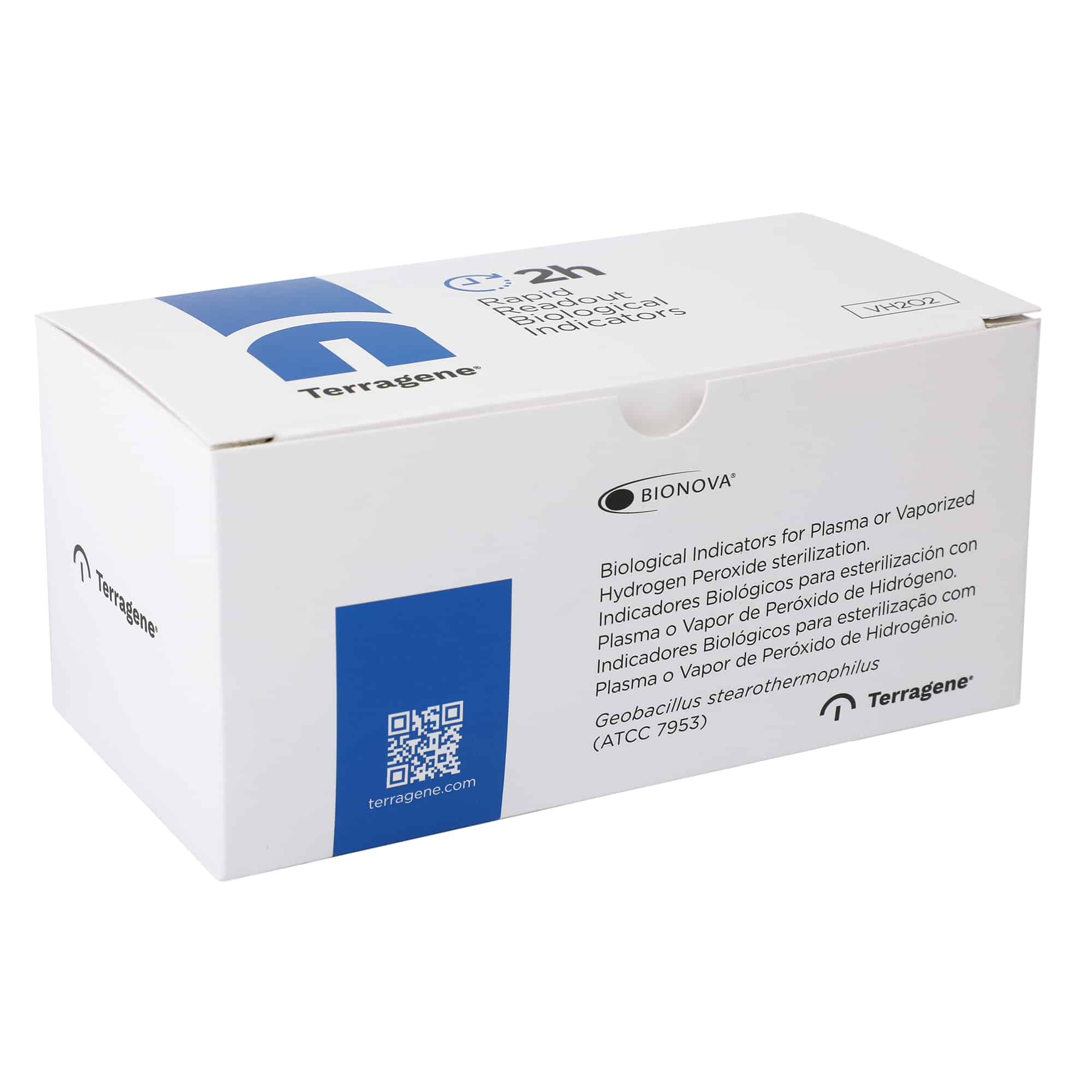
2h Biological Indicator for Hydrogen Peroxide
Additional information
| Brand | Bionova |
|---|---|
| Process | Hydrogen Peroxide |
| Packaging | 50 |
| Microorganism | Geobacillus stearothermophilus (ATCC® 7953) |
| Population | 10^6 Spores/carrier |
| Read-Out Time | 2 h |
| Regulations | IRAM 37102-1, ISO 11138-1 |
| Field of activity | Healthcare |
Description
Rapid Self-contained Biological Indicator for Plasma or Vaporized Hydrogen Peroxide sterilization processes.Geobacillus stearothermophilus (ATCC® 7953) 10^6 spores per vial. Readout: 2 hours.
FDA cleared
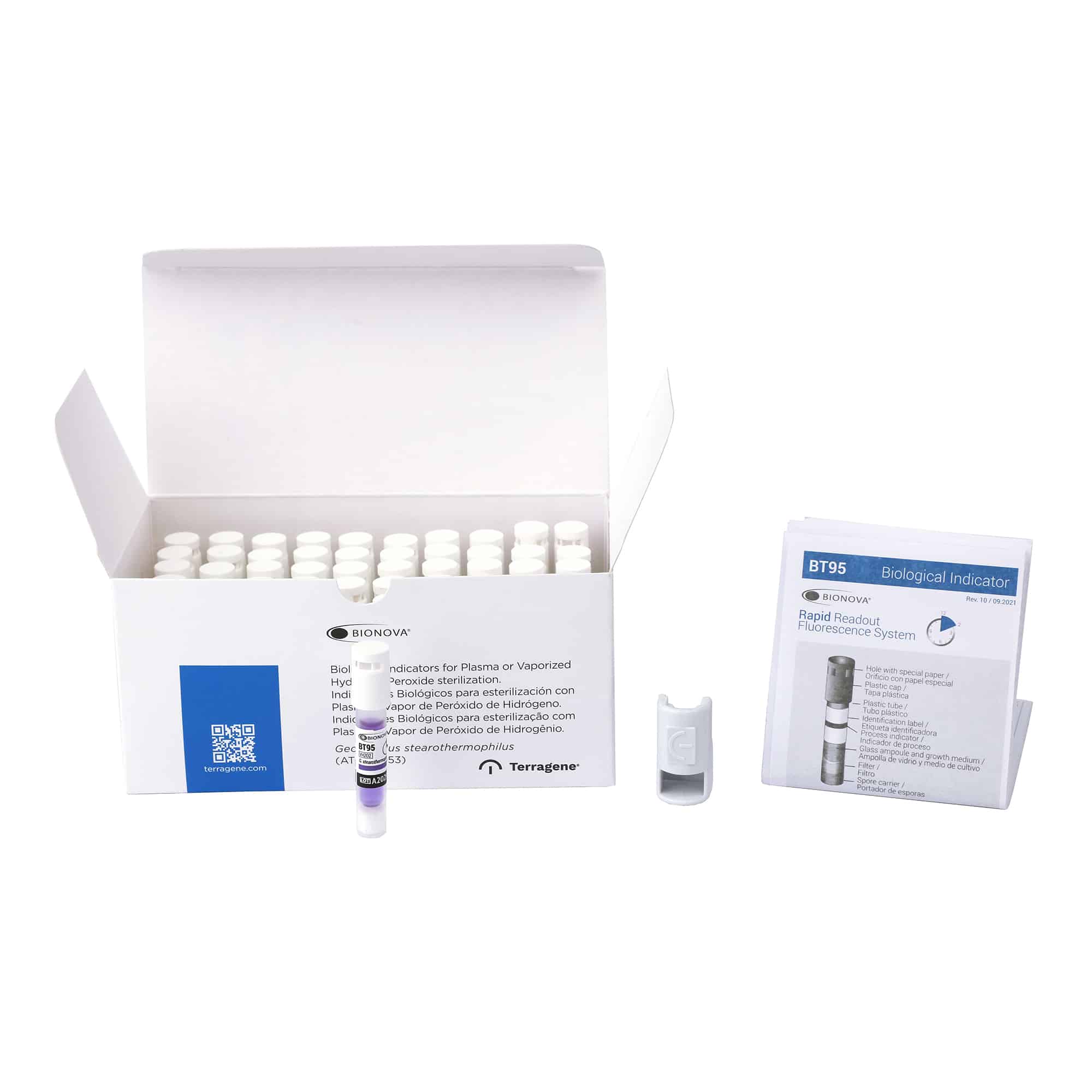
Terragene® Bionova® BT95 Fluorescence Rapid Readout Biological Indicators are single-use Self-Contained Biological Indicators (SCBIs) that consist of a polypropylene tube, a spore carrier and a glass ampoule with a culture medium, enclosed with a colored cap.
Each tube contains a population of Geobacillus stearotermophilus ATCC® 7953 spores inoculated on a spore carrier, a plastic cap with holes and a barrier permeable to Plasma or Vaporized Hydrogen Peroxide. Each BT95 has a Process Indicator on label that changes from purple to green when exposed to hydrogen peroxide.
Please review the instructions for use for applicable cycles in your country
Instructions for Use
1. Identify the Terragene® Bionova® BT95 SCBI by writing the sterilizer number (in case of having more than one), load number and processing date on the label.
2. Pack the SCBI along with materials to be sterilized in an appropriate package according to recommended sterilization practices. Place the package in those areas which are considered most inaccessible for the sterilizing agent (e.g., the center of the load and areas near the door).
3. Sterilize as usual.
4. After the sterilization process has finished, open the sterilizer door, wait five minutes and remove the SCBI from the package. CAUTION: Wear safety glasses and gloves when removing Terragene® Bionova® BT95 SCBI from the sterilized package.
WARNING: Do not crush or handle the SCBI excessively, since this might cause the glass ampoule to burst. Let the SCBI cool down until it reaches room temperature.
5. Check the Process Indicator on SCBI label. A color change to green indicates that the SCBI has been exposed to Hydrogen Peroxide.
IMPORTANT: This color change does not evidence the process effectiveness to achieve sterility. If the Process Indicator color has not changed, check the sterilization process.
6. Press the lid to seal the tube. Crush the ampoule contained in the SCBI with an individual ampoule crusher or with the ampoule crusher placed within the incubator´s incubation area. Then shake the tube down vigorously, with movements similar to those performed to lower the temperature in a mercury thermometer, until the medium reaches the base of the tube and soaks the spore carrier entirely. Finally, place the SCBI in the incubator.
IMPORTANT: Use a non-sterilized SCBI as a positive control in order to ensure that correct incubation conditions were met; the capability of medium to promote rapid growth; that viability of spores has not been altered due to improper storage temperature, humidity or proximity to chemicals and proper functioning of Terragene® Bionova® Auto-Reader Incubators. Both, the positive control indicator and the processed indicator, should belong to the same batch.
7. Incubate the processed indicator and the positive control indicator in the appropriate Terragene® Bionova® Auto-Reader incubators for a maximum of 2 hours at (60 ± 2) °C for rapid readout.
NOTE: Holding time between sterilization and incubation should not exceed a 7-day period. Fluorescence detection by the reader (excitation 340-380 nm / emission 455-465 nm) means failure in the sterilization process. If no fluorescence is detected at 2-hour incubation, the result is negative.
Description
Rapid Self-contained Biological Indicator for Plasma or Vaporized Hydrogen Peroxide sterilization processes.Geobacillus stearothermophilus (ATCC® 7953) 10^6 spores per vial. Readout: 2 hours.
FDA cleared
Terragene® Bionova® BT95 Fluorescence Rapid Readout Biological Indicators are single-use Self-Contained Biological Indicators (SCBIs) that consist of a polypropylene tube, a spore carrier and a glass ampoule with a culture medium, enclosed with a colored cap.
Each tube contains a population of Geobacillus stearotermophilus ATCC® 7953 spores inoculated on a spore carrier, a plastic cap with holes and a barrier permeable to Plasma or Vaporized Hydrogen Peroxide. Each BT95 has a Process Indicator on label that changes from purple to green when exposed to hydrogen peroxide.
Please review the instructions for use for applicable cycles in your country
Instructions for Use
1. Identify the Terragene® Bionova® BT95 SCBI by writing the sterilizer number (in case of having more than one), load number and processing date on the label.
2. Pack the SCBI along with materials to be sterilized in an appropriate package according to recommended sterilization practices. Place the package in those areas which are considered most inaccessible for the sterilizing agent (e.g., the center of the load and areas near the door).
3. Sterilize as usual.
4. After the sterilization process has finished, open the sterilizer door, wait five minutes and remove the SCBI from the package. CAUTION: Wear safety glasses and gloves when removing Terragene® Bionova® BT95 SCBI from the sterilized package.
WARNING: Do not crush or handle the SCBI excessively, since this might cause the glass ampoule to burst. Let the SCBI cool down until it reaches room temperature.
5. Check the Process Indicator on SCBI label. A color change to green indicates that the SCBI has been exposed to Hydrogen Peroxide.
IMPORTANT: This color change does not evidence the process effectiveness to achieve sterility. If the Process Indicator color has not changed, check the sterilization process.
6. Press the lid to seal the tube. Crush the ampoule contained in the SCBI with an individual ampoule crusher or with the ampoule crusher placed within the incubator´s incubation area. Then shake the tube down vigorously, with movements similar to those performed to lower the temperature in a mercury thermometer, until the medium reaches the base of the tube and soaks the spore carrier entirely. Finally, place the SCBI in the incubator.
IMPORTANT: Use a non-sterilized SCBI as a positive control in order to ensure that correct incubation conditions were met; the capability of medium to promote rapid growth; that viability of spores has not been altered due to improper storage temperature, humidity or proximity to chemicals and proper functioning of Terragene® Bionova® Auto-Reader Incubators. Both, the positive control indicator and the processed indicator, should belong to the same batch.
7. Incubate the processed indicator and the positive control indicator in the appropriate Terragene® Bionova® Auto-Reader incubators for a maximum of 2 hours at (60 ± 2) °C for rapid readout.
NOTE: Holding time between sterilization and incubation should not exceed a 7-day period. Fluorescence detection by the reader (excitation 340-380 nm / emission 455-465 nm) means failure in the sterilization process. If no fluorescence is detected at 2-hour incubation, the result is negative.