7 sec Steam Test Pack Kit (with moving front integrator) – Photon
SKU:
KPCD225-C
Category: Process Challenge Device
Tags biological indicators, Process Challenge Device
Additional information
| Brand | |
|---|---|
| Process | Steam |
| Packaging | Kit with 25 PCDs + 25 SCBIs |
| Microorganism | Geobacillus stearothermophilus (ATCC® 7953) |
| Population | 10^6 Spores/carrier |
| Read-Out Time | 7 sec |
| Initial Color | Pink |
| Final Color | Brown |
| Regulations | ANSI/AAMI ST79, ISO 11138-3, ISO 11140-1 |
| Possible target markets |
Description
Process Challenge Device for Steam sterilization processes.
Usage
For routine load release, especially for loads containing implants. For routine sterilizer monitoring. For periodic validation of the sterilizer (after installation, repair, relocation).
Detects inadequate air removal and steam penetration in dynamic- airremoval (pre-vacuum) and gravity displacement, steam autoclaves between 132-135 ºC.
Applicable regulation
Designed under Quality Management System standards ISO 13485:2016/NS-EN ISO 13485:2016. ISO 11140-1:2014, ISO 11138-1:2017, ISO 11138-3:2017, ANSI/AAMI ST79.
Authorization
ANMAT (Argentinean National Administration of Drugs, Food and Medical Devices) PM 1614-4.
Classification
Class 1, according to risk (ANMAT).
Characteristics
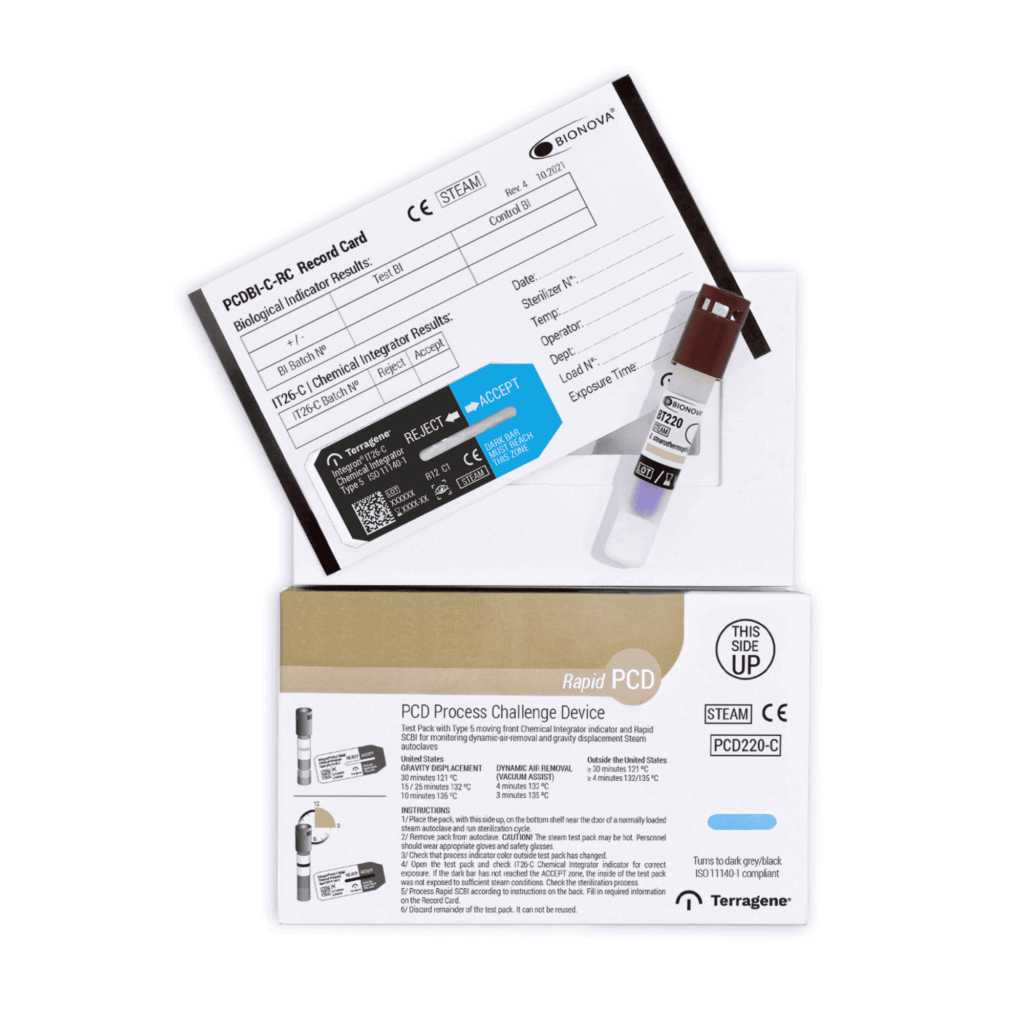
The kit consist of:
– PCD225-C: Porous cards system holding:
A Self-contained Biological Indicator Bionova® BT225 (Photon).
A Type 5 chemical indicator Integron® IT26-C (moving-front
integrator).
A self-adhesive Record Card, PCDBI-C-RC, where data on sterilization
cycle may be written.
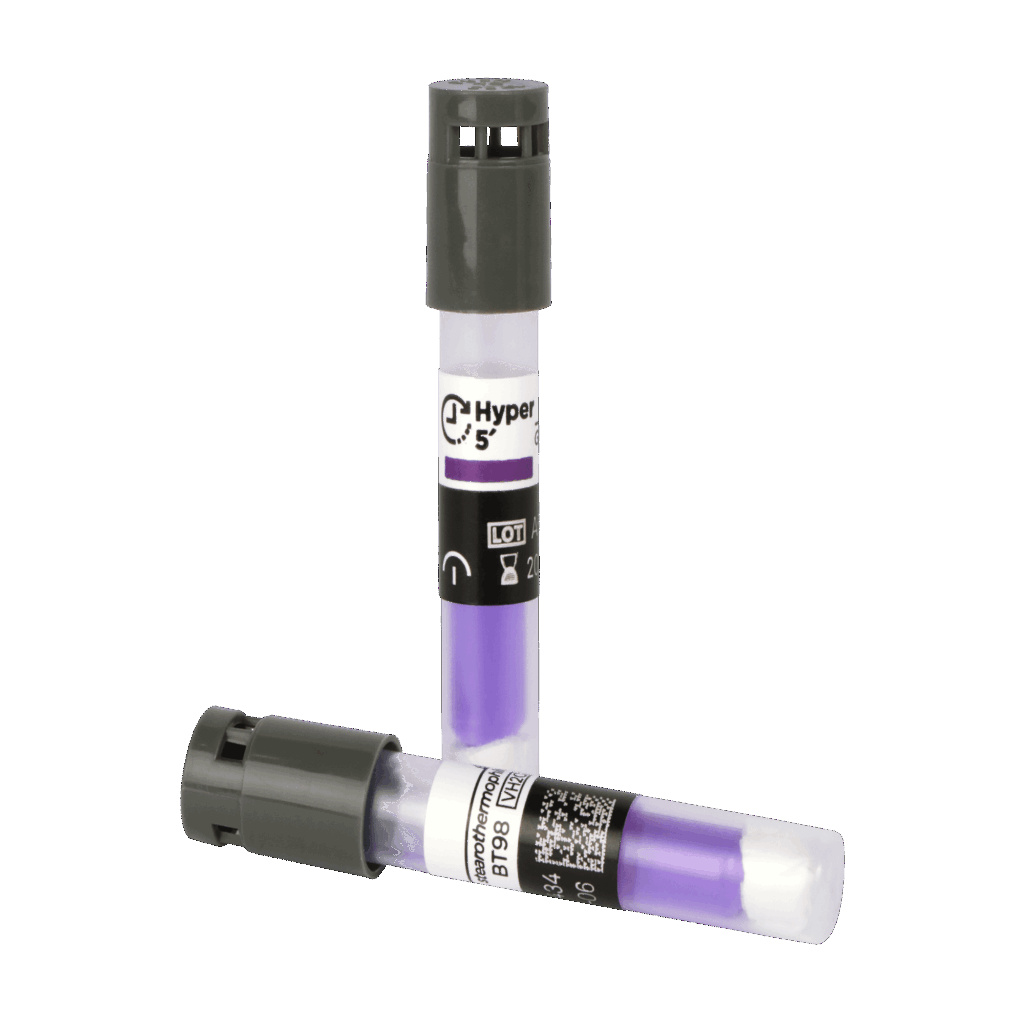
– Self-contained Biological Indicator Bionova® BT225 (to be used as a
positive control)
Process Indicator:
Initial color: light blue
Final color: dark grey or black
IT26-C Integrator Indicator (within the challenge device): The migration is visible through a zone marked ACCEPT or REJECT, thus indicating whether sterilization conditions were met. Integration condition is calibrated against the kill time of a 106 G. stearothermophilus ATCC® (ATCC is a registered trademark of American Type Culture Collection) 7953 spores’ population, calculated in BIER (Biological Indicator Evaluator Resistometer). Conditions: saturated steam at 121 °C, 128 °C, 135 °C.
BT225 (Photon) the Instant Self-contained Biological Indicator (within the challenge device): ≥ 106 G. stearothermophilus ATCC® (ATCC is a registered trademark of American Type Culture Collection) 7953 spores per vial in paper carrier. Glass ampoule with growth indicator medium. Label with chemical indicator line printed with Steam reactive ink (Color change: pink to brown).
Final results: instant fluorescence readout (7 second readout) after incubation at 60 °C (sensitivity ≥ 97%). A visual confirmation of the result is optional (color change of the medium caused by pH change) and can be made after incubation at 60 °C for 48 hours and/or for 7 days.
NOTE: If sterilization process was not successful, indicator medium will turn yellow after incubation, thus indicating the presence of living G. stearothermophilus spores. If sterilization process was successful, indicator medium will remain purple after incubation. Environmental conditions during manufacture T= 15-30 °C, RH= 30-80 %.
Storage conditions
T= 10-30 °C, RH= 30-80 %, keep out of direct ligh Transport conditions Storage conditions should be strictly followed. Carry in closed and reinforced boxes in order to avoid damages. The transport of this product does not represent a risk for health.
Shelf life
18 months.
Packing
Presentations: 25 PCD225-C + 25 BT225 Biological Indicators per box.
25 PCD225-C + 5 BT225 Biological Indicators per box.
16 PCD225-C + 4 BT225 Biological Indicators per box. Packing information: product code and description, regulation, conditions for use, instructions for use, storage conditions, manufacturer information and data on box´s label.
Labelling
On product’s packing: product code and description, presentation, regulation, batch number, batch number of the Self-contained Biological Indicator, manufacture and expiration date, bar code and datamatrix code.
Possible target markets
Healthcare, Food, Pharmaceutical and Medical Industries.
Other important information
Read product’s instructions for use thoroughly before use.
Precautions
Do not store the product near sterilizing agents.
Do not expose this product to EO, Dry Heat, Radiation or any sterilization process other than Steam.
Description
Process Challenge Device for Steam sterilization processes.
Usage
For routine load release, especially for loads containing implants. For routine sterilizer monitoring. For periodic validation of the sterilizer (after installation, repair, relocation).
Detects inadequate air removal and steam penetration in dynamic- airremoval (pre-vacuum) and gravity displacement, steam autoclaves between 132-135 ºC.
Applicable regulation
Designed under Quality Management System standards ISO 13485:2016/NS-EN ISO 13485:2016. ISO 11140-1:2014, ISO 11138-1:2017, ISO 11138-3:2017, ANSI/AAMI ST79.
Authorization
ANMAT (Argentinean National Administration of Drugs, Food and Medical Devices) PM 1614-4.
Classification
Class 1, according to risk (ANMAT).
Characteristics
The kit consist of:
– PCD225-C: Porous cards system holding:
A Self-contained Biological Indicator Bionova® BT225 (Photon).
A Type 5 chemical indicator Integron® IT26-C (moving-front
integrator).
A self-adhesive Record Card, PCDBI-C-RC, where data on sterilization
cycle may be written.
– Self-contained Biological Indicator Bionova® BT225 (to be used as a
positive control)
Process Indicator:
Initial color: light blue
Final color: dark grey or black
IT26-C Integrator Indicator (within the challenge device): The migration is visible through a zone marked ACCEPT or REJECT, thus indicating whether sterilization conditions were met. Integration condition is calibrated against the kill time of a 106 G. stearothermophilus ATCC® (ATCC is a registered trademark of American Type Culture Collection) 7953 spores’ population, calculated in BIER (Biological Indicator Evaluator Resistometer). Conditions: saturated steam at 121 °C, 128 °C, 135 °C.
BT225 (Photon) the Instant Self-contained Biological Indicator (within the challenge device): ≥ 106 G. stearothermophilus ATCC® (ATCC is a registered trademark of American Type Culture Collection) 7953 spores per vial in paper carrier. Glass ampoule with growth indicator medium. Label with chemical indicator line printed with Steam reactive ink (Color change: pink to brown).
Final results: instant fluorescence readout (7 second readout) after incubation at 60 °C (sensitivity ≥ 97%). A visual confirmation of the result is optional (color change of the medium caused by pH change) and can be made after incubation at 60 °C for 48 hours and/or for 7 days.
NOTE: If sterilization process was not successful, indicator medium will turn yellow after incubation, thus indicating the presence of living G. stearothermophilus spores. If sterilization process was successful, indicator medium will remain purple after incubation. Environmental conditions during manufacture T= 15-30 °C, RH= 30-80 %.
Storage conditions
T= 10-30 °C, RH= 30-80 %, keep out of direct ligh Transport conditions Storage conditions should be strictly followed. Carry in closed and reinforced boxes in order to avoid damages. The transport of this product does not represent a risk for health.
Shelf life
18 months.
Packing
Presentations: 25 PCD225-C + 25 BT225 Biological Indicators per box.
25 PCD225-C + 5 BT225 Biological Indicators per box.
16 PCD225-C + 4 BT225 Biological Indicators per box. Packing information: product code and description, regulation, conditions for use, instructions for use, storage conditions, manufacturer information and data on box´s label.
Labelling
On product’s packing: product code and description, presentation, regulation, batch number, batch number of the Self-contained Biological Indicator, manufacture and expiration date, bar code and datamatrix code.
Possible target markets
Healthcare, Food, Pharmaceutical and Medical Industries.
Other important information
Read product’s instructions for use thoroughly before use.
Precautions
Do not store the product near sterilizing agents.
Do not expose this product to EO, Dry Heat, Radiation or any sterilization process other than Steam.
HAVE A QUESTION ABOUT OUR PRODUCTS?
WE´RE HERE TO HELP.