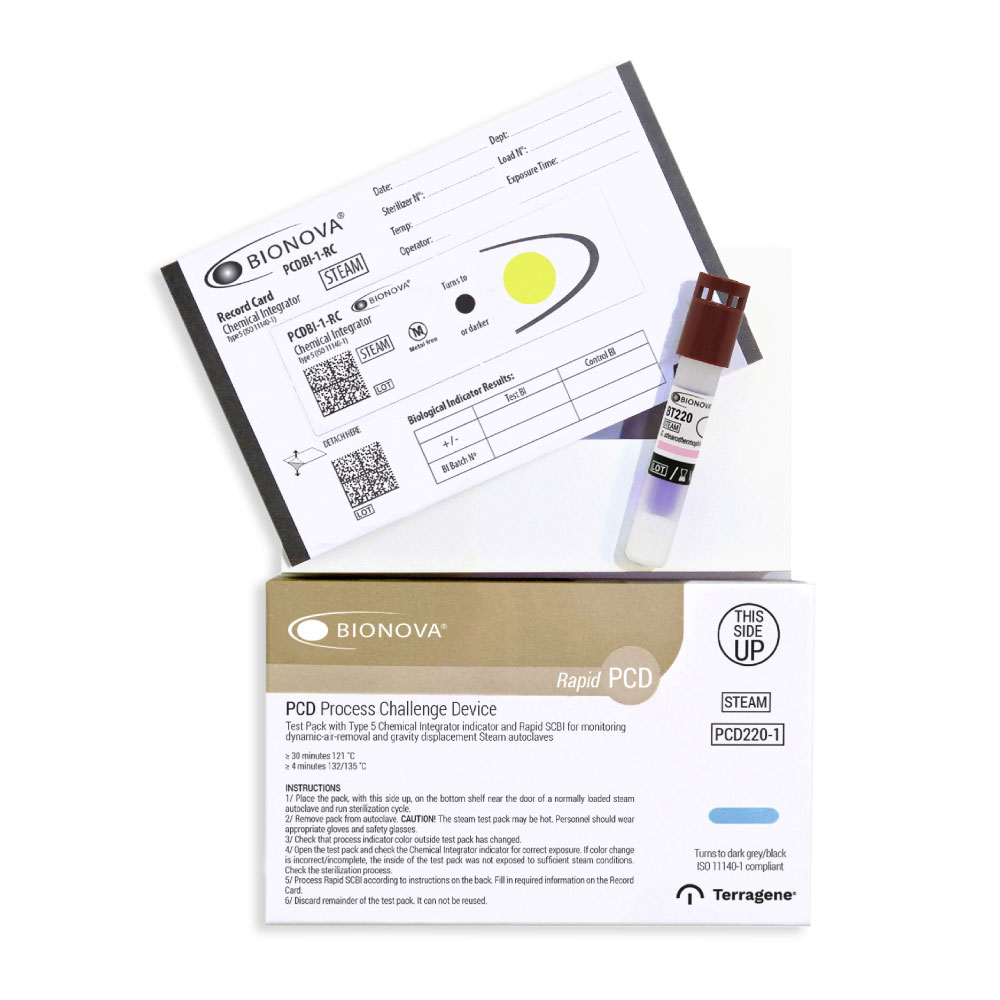
3h Steam Test Pack (with one point integrator – yellow)
Additional information
| Brand | |
|---|---|
| Process | Steam |
| Packaging | Single PCD |
| Microorganism | Geobacillus stearothermophilus (ATCC® 7953) |
| Population | 10^7 Spores/carrier |
| Read-Out Time | 3 h |
| Regulations | ANSI/AAMI ST79, ISO 11138-3, ISO 11140-1 |
| Possible target markets |
Description
PCD220-1 / Process Challenge Device for Steam sterilization processes with self-adhesive Type 5 Indicator (Turns from Yellow to Black) and Bionova® Rapid Self-contained Biological Indicator BT220.
FDA cleared
For routine load release, especially for loads containing implants. For routine sterilizer monitoring. For periodic validation of the sterilizer (after installation, repair, relocation).
Detects inadequate air removal and steam penetration in dynamic-airremoval (pre-vacuum) and gravity displacement steam autoclaves.
Instructions for use
1. Place the pack inside a steam autoclave with the load to be sterilized. Place it in areas that you consider a priori most inaccessible for the sterilizing agent (steam). Generally, the problematic areas are the center of the load and the bottom shelf near the door and over the drain.
2. Run sterilization cycle.
3. After the sterilization process has finished, open the sterilizer door, wait for 5 minutes and remove the test pack. NOTE: The color of the box design may vary from the original color after undergoing the sterilization cycle. This does not represent a problem regarding the operation or quality of the product.
4. Check that the external process indicator color on the outside of the test pack has changed. Open the test pack, wait 5 minutes and remove the SCBI. Allow it to cool down to room temperature. PRECAUTION! Wear safety glasses and gloves when removing the biological indicator from the sterilized test pack. WARNING! Do not crush or handle the biological indicator excessively, since this might cause the glass ampoule to burst.
5. Check the chemical integrator on the Record Card for correct exposure. Color change to the reference confirms that the inside of the pack has been exposed to sufficient sterilization conditions. Otherwise, check the sterilization process.
6. Check the process indicator on the label of the biological indicator. A color change to brown confirms that the biological indicator has been exposed to steam.
IMPORTANT: This color change does not indicate that the process was sufficient to achieve sterility.
7. Identify the Bionova® BT220 SCBI by writing the sterilizer number (in case of having more than one sterilizer), load number, and processing date on the label. Fill out the required information on the Record Card.
8. Crush the glass ampoule contained in the SCBI. Then shake the tube down vigorously, with movements similar to those performed to lower the temperature in a mercury thermometer, until the medium reaches the base of the tube and soaks the spores carrier entirely. Incubate at (60 ± 2) ºC in an appropriate Bionova® auto-reader incubator.
IMPORTANT: Use a non-sterilized biological indicator as a positive control whenever a processed biological indicator is incubated. The positive control ensures that appropriate incubation conditions are met. Both the positive control indicator and the processed indicator should belong to the same batch. The indicator used as positive control must yield a positive result.
9. Incubate the processed biological indicator and the indicator used as positive control for 3 hours at (60 ± 2) °C to get the final fluorescence result. A 48-hour readout by visual color change is optional to confirm the 3-hour result. A positive fluorescence result (or growth medium color change after the 48-hour optional incubation) of the processed biological indicator means a sterilization process failure has occurred. If a negative fluorescence result is obtained (or growth medium remains the original color after the optional incubation period) the sterilization process was satisfactory.
10. Wait for the final results. Fill out the SCBI results and adhere the self-adhesive whole Record Card or alternatively, only the area containing the integrator indicator. WARNING! Do not use the sterilizer until the biological indicator test results are negative.
11. Discard the test pack and the SCBI immediately.
Description
PCD220-1 / Process Challenge Device for Steam sterilization processes with self-adhesive Type 5 Indicator (Turns from Yellow to Black) and Bionova® Rapid Self-contained Biological Indicator BT220.
FDA cleared
For routine load release, especially for loads containing implants. For routine sterilizer monitoring. For periodic validation of the sterilizer (after installation, repair, relocation).
Detects inadequate air removal and steam penetration in dynamic-airremoval (pre-vacuum) and gravity displacement steam autoclaves.
Instructions for use
1. Place the pack inside a steam autoclave with the load to be sterilized. Place it in areas that you consider a priori most inaccessible for the sterilizing agent (steam). Generally, the problematic areas are the center of the load and the bottom shelf near the door and over the drain.
2. Run sterilization cycle.
3. After the sterilization process has finished, open the sterilizer door, wait for 5 minutes and remove the test pack. NOTE: The color of the box design may vary from the original color after undergoing the sterilization cycle. This does not represent a problem regarding the operation or quality of the product.
4. Check that the external process indicator color on the outside of the test pack has changed. Open the test pack, wait 5 minutes and remove the SCBI. Allow it to cool down to room temperature. PRECAUTION! Wear safety glasses and gloves when removing the biological indicator from the sterilized test pack. WARNING! Do not crush or handle the biological indicator excessively, since this might cause the glass ampoule to burst.
5. Check the chemical integrator on the Record Card for correct exposure. Color change to the reference confirms that the inside of the pack has been exposed to sufficient sterilization conditions. Otherwise, check the sterilization process.
6. Check the process indicator on the label of the biological indicator. A color change to brown confirms that the biological indicator has been exposed to steam.
IMPORTANT: This color change does not indicate that the process was sufficient to achieve sterility.
7. Identify the Bionova® BT220 SCBI by writing the sterilizer number (in case of having more than one sterilizer), load number, and processing date on the label. Fill out the required information on the Record Card.
8. Crush the glass ampoule contained in the SCBI. Then shake the tube down vigorously, with movements similar to those performed to lower the temperature in a mercury thermometer, until the medium reaches the base of the tube and soaks the spores carrier entirely. Incubate at (60 ± 2) ºC in an appropriate Bionova® auto-reader incubator.
IMPORTANT: Use a non-sterilized biological indicator as a positive control whenever a processed biological indicator is incubated. The positive control ensures that appropriate incubation conditions are met. Both the positive control indicator and the processed indicator should belong to the same batch. The indicator used as positive control must yield a positive result.
9. Incubate the processed biological indicator and the indicator used as positive control for 3 hours at (60 ± 2) °C to get the final fluorescence result. A 48-hour readout by visual color change is optional to confirm the 3-hour result. A positive fluorescence result (or growth medium color change after the 48-hour optional incubation) of the processed biological indicator means a sterilization process failure has occurred. If a negative fluorescence result is obtained (or growth medium remains the original color after the optional incubation period) the sterilization process was satisfactory.
10. Wait for the final results. Fill out the SCBI results and adhere the self-adhesive whole Record Card or alternatively, only the area containing the integrator indicator. WARNING! Do not use the sterilizer until the biological indicator test results are negative.
11. Discard the test pack and the SCBI immediately.
HAVE A QUESTION ABOUT OUR PRODUCTS?
WE´RE HERE TO HELP.