Chemical Integrator for EtO
Additional information
| Brand | |
|---|---|
| Process | Ethylene Oxide |
| Packaging | 250 |
| Initial Color | Level 1 Red, Level 2 Purple-Brown |
| Final Color | Level 1 Yellow, Level 2 Green |
| Indicator Type | Type 5 Chemical Indicator |
| Regulations | ISO 11140-1 |
| Possible target markets |
Description
2 level chemical integrator for Ethylene Oxide sterilization processes. One-side laminated strips. Metals free.
FDA cleared
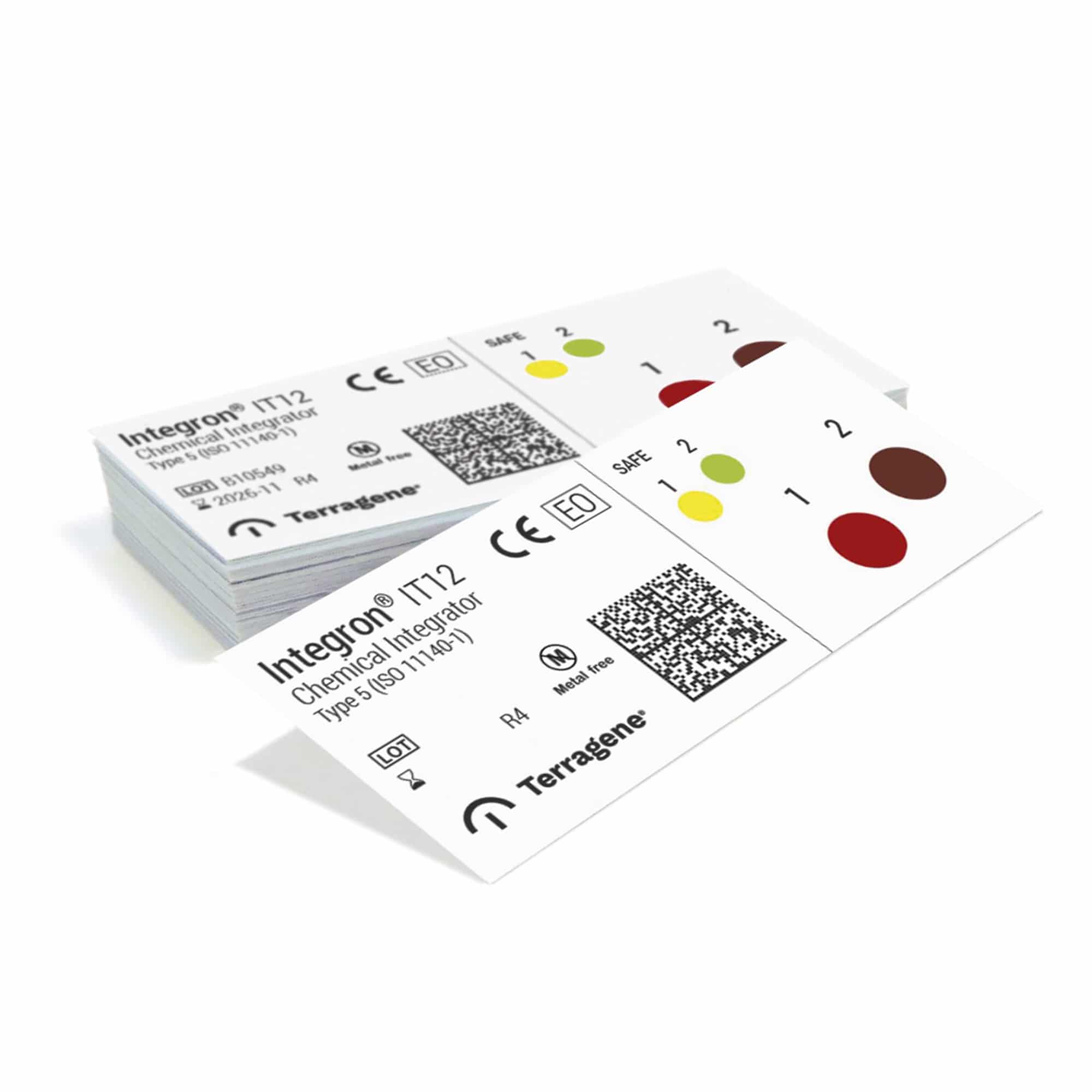
Integron® IT12 Chemical Integrators (Type 5 according to ISO 11140-1:2014 standard) are single-use two level chemical indicators that consist of paper strips laminated on the back side and printed with indicator ink.
They have been designed for monitoring Ethylene Oxide (EO) sterilization processes. The integration level contains a purple/brown ink which was developed to turn to green when a theoretical spore population reaches its kill time, thus indicating that the SAFE condition has been met.
Level 1 – Red: Exposure level. Indicator ink turns yellow to indicate exposition to the process, but does not ensure that the variables required to achieve the integration condition have been achieved (UNSAFE condition).
Level 2 – Purple / brown: Integration level. Indicator ink turns green to indicate that the integration condition indicated in the quality certificate has been achieved. This condition consists of the kill time of a theoretical 10^6 Bacillus atrophaeus spore population, calculated in a BIER (Biological Indicator Evaluator Resistometer), and is called SAFE condition. Indicators 100% free of toxic heavy metals.
Please review the instructions for use for applicable cycles in your country
Instructions for Use
1. Place a Integron® IT12 Chemical Integrator along with the material to be sterilized in a suitable package according to the recommended sterilization techniques. Place the indicators in those areas of most difficult access to the sterilizing agent.
2. Perform the sterilization cycle.
3. After the sterilization process has finished and evacuation/ventilation process is over, you may:
A. Remove the integrator indicator taking the necessary precaution to avoid contact with the remaining sterilizing agent.
B. Ventilate the load together with the integrator indicator and then perform the reading.
4. Analyze the results. If the SAFE integration condition has been reached, the sterilization process has been effective.
Description
2 level chemical integrator for Ethylene Oxide sterilization processes. One-side laminated strips. Metals free.
FDA cleared
Integron® IT12 Chemical Integrators (Type 5 according to ISO 11140-1:2014 standard) are single-use two level chemical indicators that consist of paper strips laminated on the back side and printed with indicator ink.
They have been designed for monitoring Ethylene Oxide (EO) sterilization processes. The integration level contains a purple/brown ink which was developed to turn to green when a theoretical spore population reaches its kill time, thus indicating that the SAFE condition has been met.
Level 1 – Red: Exposure level. Indicator ink turns yellow to indicate exposition to the process, but does not ensure that the variables required to achieve the integration condition have been achieved (UNSAFE condition).
Level 2 – Purple / brown: Integration level. Indicator ink turns green to indicate that the integration condition indicated in the quality certificate has been achieved. This condition consists of the kill time of a theoretical 10^6 Bacillus atrophaeus spore population, calculated in a BIER (Biological Indicator Evaluator Resistometer), and is called SAFE condition. Indicators 100% free of toxic heavy metals.
Please review the instructions for use for applicable cycles in your country
Instructions for Use
1. Place a Integron® IT12 Chemical Integrator along with the material to be sterilized in a suitable package according to the recommended sterilization techniques. Place the indicators in those areas of most difficult access to the sterilizing agent.
2. Perform the sterilization cycle.
3. After the sterilization process has finished and evacuation/ventilation process is over, you may:
A. Remove the integrator indicator taking the necessary precaution to avoid contact with the remaining sterilizing agent.
B. Ventilate the load together with the integrator indicator and then perform the reading.
4. Analyze the results. If the SAFE integration condition has been reached, the sterilization process has been effective.
HAVE A QUESTION ABOUT OUR PRODUCTS?
WE´RE HERE TO HELP.