ATP testing was born to serve a specific purpose in the food industry market: to
monitor microbiological possible contaminations after washing/disinfection of
the surfaces and machines. After this, and as consequence of healthcare
acquired infections outbreaks a few decades ago, hospitals started to perceive
that they need to incorporate a similar control inside the SPD/CSSD. Since there
was no better technology or testing method available rather than ATP at that
time, both hospital’s urge and manufacturer’s smartness, led the market to
incorporate this testing method inside hospitals.
However ATP systems does not seem to be the best choice for this specific
purpose. And below a list of reasons why:
• The main objective of a washing/cleaning process in hospitals is to eliminate blood and tissue remainings, thus guaranteeing that subsequent disinfection and sterilization processes will be successful. Nowadays, the main challenge that washer and detergent manufacturers face, is the complete removal of proteins from reprocessed instruments. Thus, if we are talking about a test for cleaning effectiveness, protein should be the chosen one, because, in biological samples, there is nothing more adherent than proteins. In this sense, blood and tissue residues consist of different cell types and free proteins. The most diffcult to remove are the proteins that coagulate and adhere to the instruments, making the washing process very di_cult to accomplish successfully. So, why bothering to measure ATP levels if instruments will subsequently be exposed to a sterilization process to remove any living cell remaining? This points out ATP detection is mainly recommended for disinfection control in places like the food industry.
• ATP does not represent a contamination itself, so microbial or organic contamination will always be an indirect measure (when measured by ATP). On the other hand, protein residues do represent a direct measure of organic/microbial contamination. Not only this, but more importantly, proteins are the most diffcult to remove residues during a cleaning process. For this reason, if we are talking about cleaning effectiveness, protein testing is undoubtedly the best marker.
• ATP is a very easy to hydrolyse molecule, so it does not represent a challenge to the washing procedure. In this sense, hospital washing/cleaning parameters do guarantee that any ATP molecule will be destroyed during the process (temperature, detergents or disinfectants, etc). Not only this, but if there is any chance that ATP molecules remain after the washing cycle, free ATP will inevitably hydrolyse within a few minutes. Consequently, it is very unlikely that an ATP test will give a significant result after a hospital washing cycle.
• It has been proved that some ATP tests fail to lyse some bacterial cells, thus the release of ATP for later detection may be compromised. (if we consider ATP as a microbial testing). Again, for instruments that will undergo a sterilization process after cleaning/disinfection, it would not be the best recommendation to test for bacterial contamination.
• It has also been shown that some detergents and disinfectants used in hospitals interfere with the bioluminescence reaction.
• ATP is not present in viruses and, more importantly nowadays, in Prions, protein infectious agents composed entirely of a protein material that causes transmissible spongiform encephalopathies, such as variant Creutzfeldt-Jakob disease. Remarkably, proteins are one of the main components of viruses, while prions actually are infectious proteins themselves. The relevance of this issue is such that institutions such as the UK Department of Health have set limits on micrograms (ug) of protein to consider an instrument as “clean” in The Health Technical Memorandum 01-01 (HTM 01-01). Therefore it is not enough with the simple detection of proteins, but also its quantification by electronic systems of high sensitivity and temporary analysis of the evaluated samples is important.
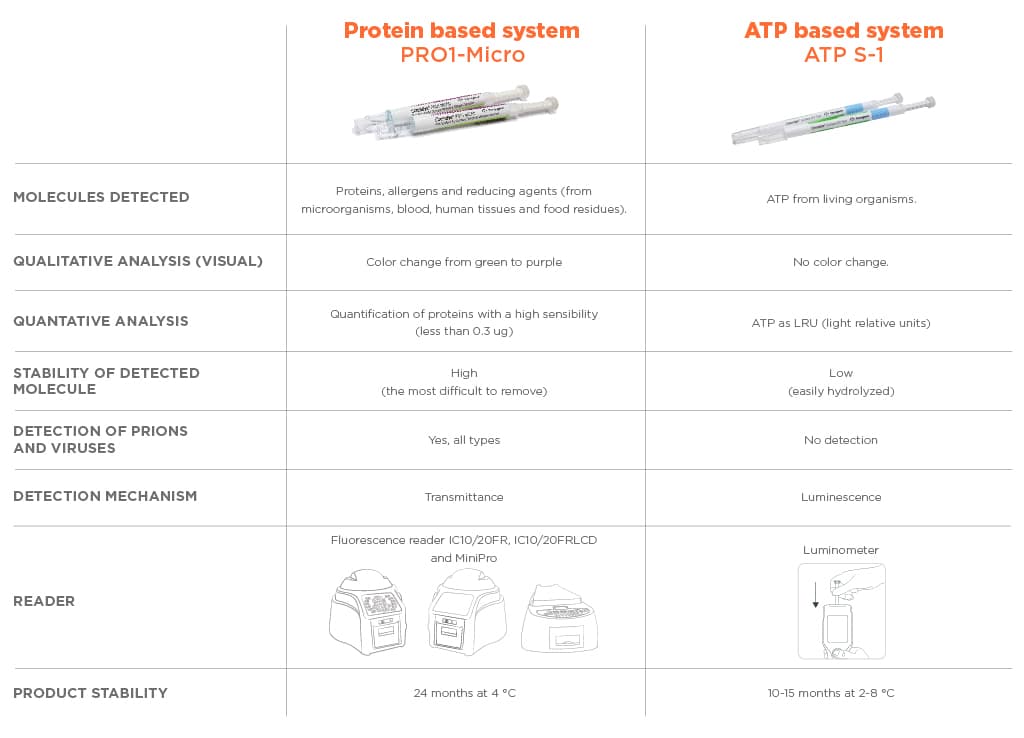
Chemdye® PRO1 MICRO represents an easy option to monitor the cleaning of reusable medical devices in the context of medical, dental practices and industry from the detection and quantification of surface proteins, allergens and reducing agents. The system consists of a swab of special characteristics and two reactive solutions contained in the same device. PRO1 MICRO offers a quantitative colorimetric result that can be measured with high sensitivity using any of the Bionova® Auto-readers: IC10/20FR, IC10/20FRLCD and MiniPro.
For the detection of microorganisms by means of ATP; the available system is Chemdye® ATP-S1. This product is similar to PRO1 MICRO in its structure; but the biochemical reactions are completely di_erent. In fact this one is based on an enzymatic reaction. ATP, the energy source of microorganisms, triggers a light reaction that can be measured in a Luminometer. It is worth to note that this system does not recognize proteins of any nature.
In conclusion, proteins do fulfil all the requirements to be the marker molecule for cleaning effectiveness monitoring. In fact, today our Chemdye® PRO1 MICRO system has become the most reliable cleaning monitoring system in the market. Additionally now with Chemdye® ATP-S1 the users can also access to highly sensitive ATP detection system to fulfill their entire requirements related to microbial contamination assessment.
• The main objective of a washing/cleaning process in hospitals is to eliminate blood and tissue remainings, thus guaranteeing that subsequent disinfection and sterilization processes will be successful. Nowadays, the main challenge that washer and detergent manufacturers face, is the complete removal of proteins from reprocessed instruments. Thus, if we are talking about a test for cleaning effectiveness, protein should be the chosen one, because, in biological samples, there is nothing more adherent than proteins. In this sense, blood and tissue residues consist of different cell types and free proteins. The most diffcult to remove are the proteins that coagulate and adhere to the instruments, making the washing process very di_cult to accomplish successfully. So, why bothering to measure ATP levels if instruments will subsequently be exposed to a sterilization process to remove any living cell remaining? This points out ATP detection is mainly recommended for disinfection control in places like the food industry.
• ATP does not represent a contamination itself, so microbial or organic contamination will always be an indirect measure (when measured by ATP). On the other hand, protein residues do represent a direct measure of organic/microbial contamination. Not only this, but more importantly, proteins are the most diffcult to remove residues during a cleaning process. For this reason, if we are talking about cleaning effectiveness, protein testing is undoubtedly the best marker.
• ATP is a very easy to hydrolyse molecule, so it does not represent a challenge to the washing procedure. In this sense, hospital washing/cleaning parameters do guarantee that any ATP molecule will be destroyed during the process (temperature, detergents or disinfectants, etc). Not only this, but if there is any chance that ATP molecules remain after the washing cycle, free ATP will inevitably hydrolyse within a few minutes. Consequently, it is very unlikely that an ATP test will give a significant result after a hospital washing cycle.
• It has been proved that some ATP tests fail to lyse some bacterial cells, thus the release of ATP for later detection may be compromised. (if we consider ATP as a microbial testing). Again, for instruments that will undergo a sterilization process after cleaning/disinfection, it would not be the best recommendation to test for bacterial contamination.
• It has also been shown that some detergents and disinfectants used in hospitals interfere with the bioluminescence reaction.
• ATP is not present in viruses and, more importantly nowadays, in Prions, protein infectious agents composed entirely of a protein material that causes transmissible spongiform encephalopathies, such as variant Creutzfeldt-Jakob disease. Remarkably, proteins are one of the main components of viruses, while prions actually are infectious proteins themselves. The relevance of this issue is such that institutions such as the UK Department of Health have set limits on micrograms (ug) of protein to consider an instrument as “clean” in The Health Technical Memorandum 01-01 (HTM 01-01). Therefore it is not enough with the simple detection of proteins, but also its quantification by electronic systems of high sensitivity and temporary analysis of the evaluated samples is important.
Chemdye® PRO1 MICRO represents an easy option to monitor the cleaning of reusable medical devices in the context of medical, dental practices and industry from the detection and quantification of surface proteins, allergens and reducing agents. The system consists of a swab of special characteristics and two reactive solutions contained in the same device. PRO1 MICRO offers a quantitative colorimetric result that can be measured with high sensitivity using any of the Bionova® Auto-readers: IC10/20FR, IC10/20FRLCD and MiniPro.
For the detection of microorganisms by means of ATP; the available system is Chemdye® ATP-S1. This product is similar to PRO1 MICRO in its structure; but the biochemical reactions are completely di_erent. In fact this one is based on an enzymatic reaction. ATP, the energy source of microorganisms, triggers a light reaction that can be measured in a Luminometer. It is worth to note that this system does not recognize proteins of any nature.
In conclusion, proteins do fulfil all the requirements to be the marker molecule for cleaning effectiveness monitoring. In fact, today our Chemdye® PRO1 MICRO system has become the most reliable cleaning monitoring system in the market. Additionally now with Chemdye® ATP-S1 the users can also access to highly sensitive ATP detection system to fulfill their entire requirements related to microbial contamination assessment.