TerraBlog

SPD/CSSD Hygiene Monitoring Systems: Protein & ATP Tests.
ATP testing was born to serve a specific purpose in the food industry market: to
monitor microbiological possible contaminations after washing/disinfection of
the surfaces and machines. After this, and as consequence of healthcare
acquired infections outbreaks a few decades ago, hospitals started to perceive
that they need to incorporate a similar control inside the SPD/CSSD. Since there
was no better technology or testing method available rather than ATP at that
time, both hospital’s urge and manufacturer’s smartness, led the market to
incorporate this testing method inside hospitals.
However ATP systems does not seem to be the best choice for this specific
purpose. And below a list of reasons why:
• The main objective of a washing/cleaning process in hospitals is to eliminate
blood and tissue remainings, thus guaranteeing that subsequent disinfection
and sterilization processes will be successful. Nowadays, the main challenge that
washer and detergent manufacturers face, is the complete removal of proteins
from reprocessed instruments. Thus, if we are talking about a test for cleaning
effectiveness, protein should be the chosen one, because, in biological samples,
there is nothing more adherent than proteins. In this sense, blood and tissue
residues consist of different cell types and free proteins. The most diffcult to
remove are the proteins that coagulate and adhere to the instruments, making
the washing process very di_cult to accomplish successfully. So, why bothering
to measure ATP levels if instruments will subsequently be exposed to a sterilization process to remove any living cell remaining? This points out ATP detection is mainly recommended for disinfection control in places like the food industry.
• ATP does not represent a contamination itself, so microbial or organic
contamination will always be an indirect measure (when measured by ATP). On
the other hand, protein residues do represent a direct measure of
organic/microbial contamination. Not only this, but more importantly, proteins
are the most di_cult to remove residues during a cleaning process. For this
reason, if we are talking about cleaning effectiveness, protein testing is
undoubtedly the best marker.
• ATP is a very easy to hydrolyse molecule, so it does not represent a challenge
to the washing procedure. In this sense, hospital washing/cleaning parameters
do guarantee that any ATP molecule will be destroyed during the process
(temperature, detergents or disinfectants, etc). Not only this, but if there is any
chance that ATP molecules remain after the washing cycle, free ATP will
inevitably hydrolyse within a few minutes. Consequently, it is very unlikely that an
ATP test will give a significant result after a hospital washing cycle.
• It has been proved that some ATP tests fail to lyse some bacterial cells, thus the
release of ATP for later detection may be compromised. (if we consider ATP as a
microbial testing). Again, for instruments that will undergo a sterilization process
after cleaning/disinfection, it would not be the best recommendation to test for
bacterial contamination.
• It has also been shown that some detergents and disinfectants used in
hospitals interfere with the bioluminescence reaction.
• ATP is not present in viruses and, more importantly nowadays, in Prions, protein
infectious agents composed entirely of a protein material that causes transmissible spongiform encephalopathies, such as variant Creutzfeldt-Jakob disease. Remarkably, proteins are one of the main components of viruses, while
prions actually are infectious proteins themselves.
The relevance of this issue is such that institutions such as the UK Department of
Health have set limits on micrograms (_g) of protein to consider an instrument
as "clean” in The Health Technical Memorandum 01-01 (HTM 01-01). Therefore it
is not enough with the simple detection of proteins, but also its quantification by
electronic systems of high sensitivity and temporary analysis of the evaluated
samples is important.
Chemdye® PRO1 MICRO represents an easy option to monitor the cleaning of
reusable medical devices in the context of medical, dental practices and industry
from the detection and quantification of surface proteins, allergens and reducing
agents. The system consists of a swab of special characteristics and two reactive
solutions contained in the same device. PRO1 MICRO offers a quantitative
colorimetric result that can be measured with high sensitivity using any of the
Bionova® Auto-readers: IC10/20FR, IC10/20FRLCD and MiniPro.
For the detection of microorganisms by means of ATP; the available system is
Chemdye® ATP-S1. This product is similar to PRO1 MICRO in its structure; but the
biochemical reactions are completely di_erent. In fact this one is based on an
enzymatic reaction. ATP, the energy source of microorganisms, triggers a light
reaction that can be measured in a Luminometer. It is worth to note that this
system does not recognize proteins of any nature.
In conclusion, proteins do fulfil all the requirements to be the marker molecule
for cleaning effectiveness monitoring. In fact, today our Chemdye® PRO1 MICRO
system has become the most reliable cleaning monitoring system in the market.
Additionally now with Chemdye® ATP-S1 the users can also access to highly
sensitive ATP detection system to fulfill their entire requirements related to
microbial contamination assessment.
Steam Sterilization Monitoring: Bionova Biological Bundle
BT224 biological indicator
Bionova BT224 is an ultra-rapid self-contained biological indicator with fluorescence technology for steam sterilization monitoring. It can monitor steam sterilization processes and obtain results in less than 20 minutes. It is a product of unprecedented quality due to several factors: the raw material it is made of; the state-of-the-art technology it features; the production processes in which this product is used; and the strict quality controls it undergoes for its release. Additionally, Bionova BT224 is a biological indicator approved by the most prestigious regulatory agencies around the world, such as the USA FDA, China CDC, and TFDA (Taiwan)
MiniBio Auto-reader
By using any Terragene Bionova Auto-readers you can obtain the result of this biological indicator. Bionova MiniBio Auto-reader is the ideal choice for reading this ultra-fast indicator due to its versatility, size, and functionality. It allows the incubation and reading of the indicator by a fluorescence reading system specially developed for Bionova biological indicators. Among its unique features, the following stand out: capability of reading any Bionova biological indicator, including for monitoring any other sterilization process (steam, H2O2, EtO, and formaldehyde), triple-detector radial reading technology, and a built-in thermal printer to facilitate the physical recording of all the results
Bionova Software
This system becomes fully established when used together with the Bionova Software. This software carries out the traceability of all the results obtained through the BT224/MiniBio combination (in addition to reading the indicator and to correlate it to the sterilization cycles). Besides coordinating the storage of data, it allows the creation of statistics associated with them, studies of the sterilizer’s performance, and reports that can be shared with different members of the institution.
The Bionova bundle (BT224, MiniBio, and traceability software) represents the most complete, fast, adequate, and precise control for monitoring steam sterilization in health care. We continue to develop science to save lives and bring well-being to communities.
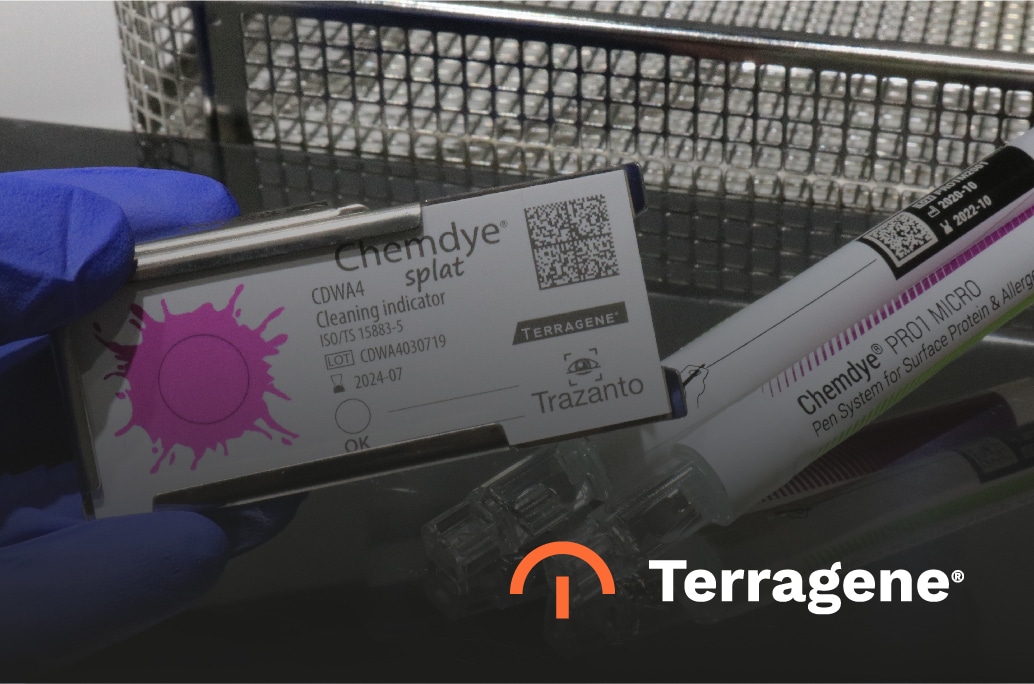
Integral Monitoring System for Washing Processes
Splat – Cleaning indicator
Splat is a specially designed product line of test soils for controlling medical material washing procedures within sterilization departments. These indicators are used together with different types of reusable stainless steel holders specifically designed to monitor washer-disinfector or ultrasonic washing machines. This unique feature allows using the same indicator strip with different holders, depending on the washing machine type (and load) to be monitored. The indicator is placed inside the holder together with the load, and the final accepted result is obtained when the ink is totally removed from the strip.
Pro1 Micro – Protein quantification system
It is essential to go beyond the visual examination of the material after the washing cycle to ensure that the executed process was efficient. Different international standards like ISO 15883 and HTM01-01 recommend using a total quantification system for residual proteins that allows (from a random instrument) testing the exact remaining amount and determine if it is possible to release the entire load, taking into account the final result obtained. Such is the case of ChemDye Pro1 Micro, a device developed by Terragene in a useful pen format with a swab that consists of a reactive solution to specifically react with the remaining proteins and provide an exact measurement using the Bionova MiniPro Auto-reader.
MiniPro Auto-reader
This auto-reader allows not only to obtain a precise quantitative result through a printed ticket but also to get a complete traceability system for the hygiene monitoring process when working together with Bionova traceability software. The perfect analysis system is achieved when using Pro1Micro and MiniPro together with Bionova’s software: reading, generation of the results, constant monitoring of the washing machines tested, and report generation. This unique worldwide monitoring system has been developed using the same technology and quality bases present in Bionova’s biological indicators, which allows transferring to the washing processes the biological monitoring technology used for sterilization.
Terragene is today the only company that has managed to bring the washing monitoring process to the same level of quality and excellence as an ultra-fast biological indicator used in sterilization monitoring processes. We are taking biotechnology to another level and applying it to health in a way never seen before.

WHAT ARE PRIONS?
Over the past few years, the infection control field has been substantially transformed because of the emergence of prions, infectious agents that have revolutionized paradigms, leading to changes in washing, hygiene, disinfection and sterilization processes.
In particular, what are prions? Prion is an English acronym derived from the words “protein” and “infection”, and was coined in 1982 by Stanley B. Prusiner. The mode of action of these infectious protein particles is through the induction of a conformational change in a natural protein of the organism, PrPc, thus altering its functionality and originating an altered protein, called PrPSc, which is able to form aggregates. Prions are responsible for transmissible spongiform encephalopathies, a group of degenerative neurological diseases such as scrapie, Creutzfeldt-Jakob disease and bovine spongiform encephalopathy, also known as “mad cow disease.”
These infectious protein particles are propagated by the transmission of anomalous proteins with incorrect folding. When a prion manages to penetrate a healthy organism, it acts on the normal form of the same type of protein existing in the organism, modifying it and making it a prion. These newly formed prions can convert more proteins, causing a chain reaction that produces large amounts of the prion protein. The infected organism difficulties rely in the fact that prions do not cause a normal immune response, since it is a normal protein of the body, so it is not recognized as pathogenic.
In the recent years, this has caused prions to become the focus of study of multiple laboratories around the world and this has spread to the scientific field dedicated to infection control, especially in neurology and reprocessing of material and instruments dedicated to this health´s sector.
Sources:
https://es.wikipedia.org/wiki/Prion
https://www.cdc.gov/prions/index.html
https://www.prionalliance.org/2013/12/02/what-are-human-prion-diseases/
https://www.prionalliance.org/2013/11/26/what-are-prions/
Imagen:
https://academic.pgcc.edu/~kroberts/Lecture/Chapter%2013/prions.html

