1h Steam Test Pack PCD Kit (with moving front integrator)
Additional information
| Brand | |
|---|---|
| Process | Steam |
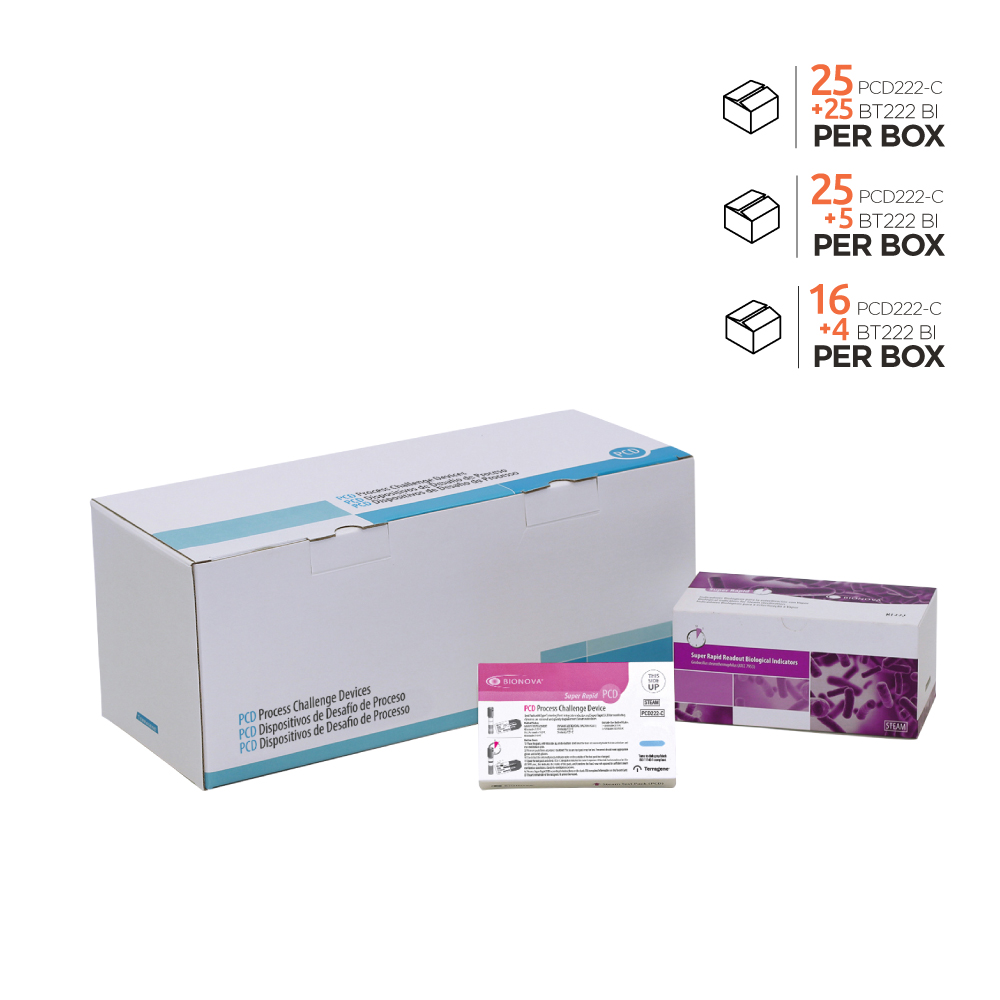
| Packaging | Kit with 25 PCDs + 25 SCBIs |
| Microorganism | Geobacillus stearothermophilus (ATCC® 7953) |
| Population | 10^6 Spores/carrier |
| Read-Out Time | 1 h |
| Regulations | ANSI/AAMI ST79, ISO 11138-1, ISO 11138-3 |
| Possible target markets |
Description
KPCD222-C / Test Pack PCD Kit. Process Challenge Device for Steam sterilization processes with moving-front Type 5 Indicator and Bionova® Super Rapid Self-contained Biological Indicator BT222, plus Bionova® BT222 Biological Indicators Positive Control.
FDA cleared
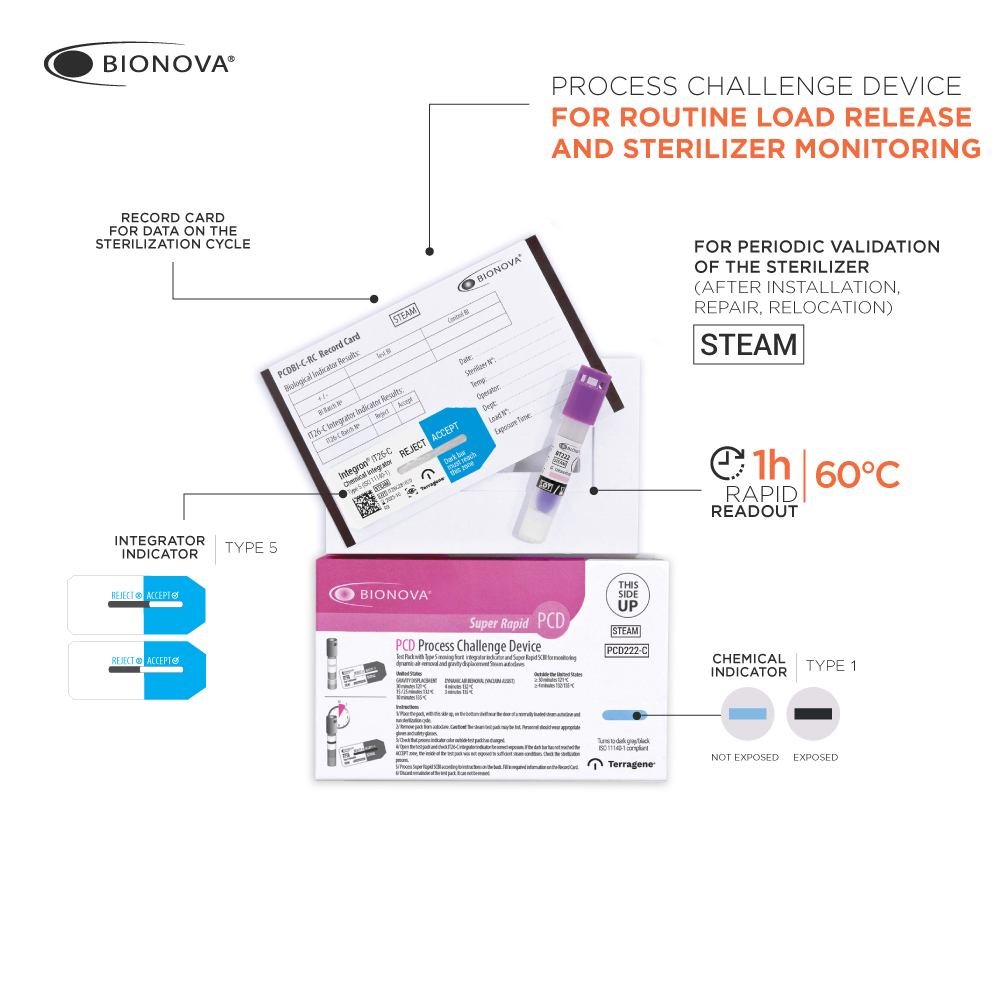
For routine load release, especially for loads containing implants. For routine sterilizer monitoring. For periodic validation of the sterilizer (after installation, repair, relocation).
Detects inadequate air removal and steam penetration in dynamic-airremoval (pre-vacuum) and gravity displacement steam autoclaves between 121-135 ºC. Please review the instructions for use for applicable cycles in your country
Characteristics
The kit consist of:
– PCD222-C: Porous cards system holding:
-A Self-contained Biological Indicator Bionova® BT222.
-A Type 5 chemical indicator Integron® IT26-C (moving-front integrator).
-A self-adhesive Record Card, PCDBI-C-RC, where data on sterilization cycle may be written.
– Self-contained Biological Indicator Bionova® BT222 (to be used as a
positive control)
Process Indicator:
Initial color: blue
Final color: dark grey or black
IT26-C Chemical Integrator (within the challenge device) The migration is visible through a zone marked ACCEPT or REJECT, thus indicating whether sterilization conditions were met. Integration condition is calibrated against the kill time of a 106 G. stearothermophilus ATCC 7953 spores€™ population, calculated in BIER (Biological Indicator Evaluator Resistometer). Conditions: saturated steam at 121 °C, 128 °C, 135 °C.
BT222 Super Rapid Self-contained Biological Indicator (within the challenge device): ‰¥106 G. stearothermophilus ATCC 7953 spores per vial in paper carrier. Glass ampoule with growth indicator medium.
Label with chemical indicator line printed with Steam reactive ink (Color change: pink to brown)
Description
KPCD222-C / Test Pack PCD Kit. Process Challenge Device for Steam sterilization processes with moving-front Type 5 Indicator and Bionova® Super Rapid Self-contained Biological Indicator BT222, plus Bionova® BT222 Biological Indicators Positive Control.
FDA cleared
For routine load release, especially for loads containing implants. For routine sterilizer monitoring. For periodic validation of the sterilizer (after installation, repair, relocation).
Detects inadequate air removal and steam penetration in dynamic-airremoval (pre-vacuum) and gravity displacement steam autoclaves between 121-135 ºC. Please review the instructions for use for applicable cycles in your country
Characteristics
The kit consist of:
– PCD222-C: Porous cards system holding:
-A Self-contained Biological Indicator Bionova® BT222.
-A Type 5 chemical indicator Integron® IT26-C (moving-front integrator).
-A self-adhesive Record Card, PCDBI-C-RC, where data on sterilization cycle may be written.
– Self-contained Biological Indicator Bionova® BT222 (to be used as a
positive control)
Process Indicator:
Initial color: blue
Final color: dark grey or black
IT26-C Chemical Integrator (within the challenge device) The migration is visible through a zone marked ACCEPT or REJECT, thus indicating whether sterilization conditions were met. Integration condition is calibrated against the kill time of a 106 G. stearothermophilus ATCC 7953 spores€™ population, calculated in BIER (Biological Indicator Evaluator Resistometer). Conditions: saturated steam at 121 °C, 128 °C, 135 °C.
BT222 Super Rapid Self-contained Biological Indicator (within the challenge device): ‰¥106 G. stearothermophilus ATCC 7953 spores per vial in paper carrier. Glass ampoule with growth indicator medium.
Label with chemical indicator line printed with Steam reactive ink (Color change: pink to brown)
HAVE A QUESTION ABOUT OUR PRODUCTS?
WE´RE HERE TO HELP.