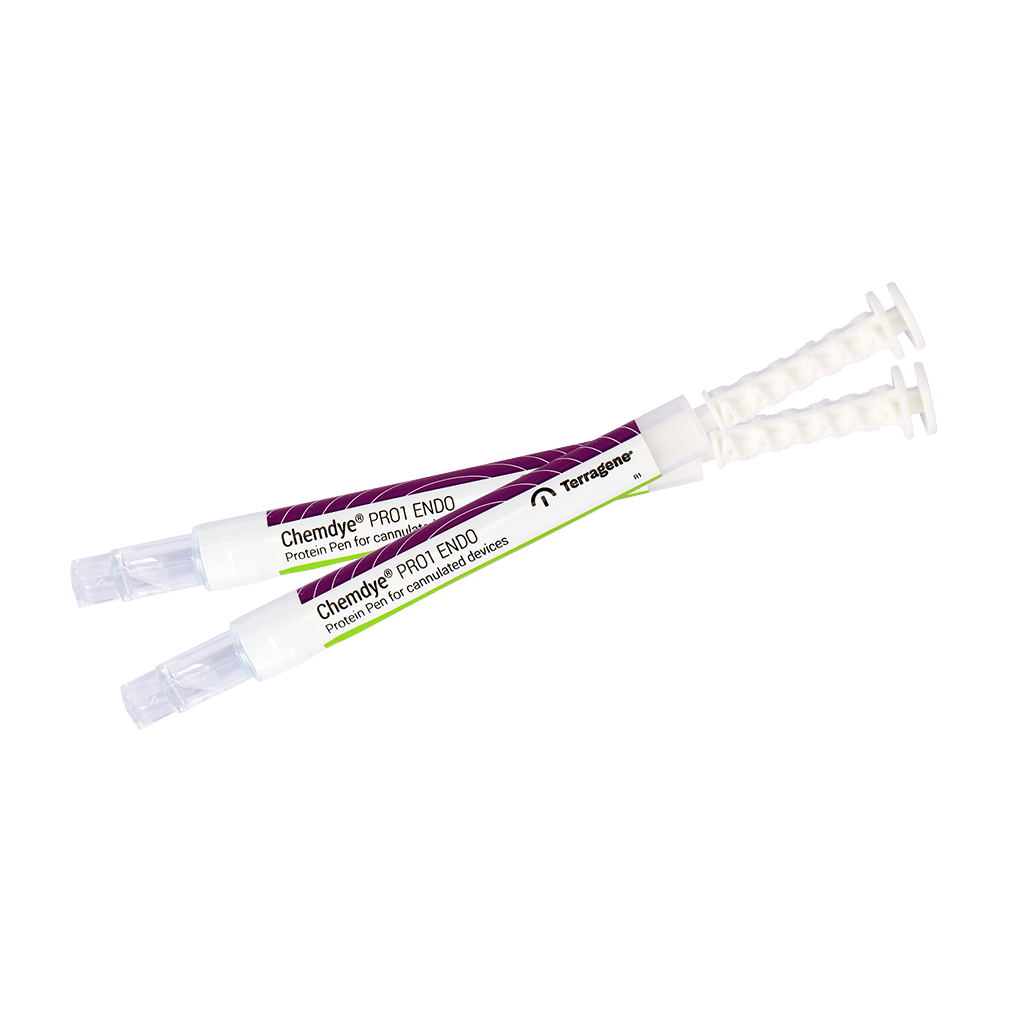
PRO1 ENDO – Protein Detection and Quantification on Cannulated Instruments
Additional information
| Brand | |
|---|---|
| Process | Hygiene Monitoring |
| Packaging | 20 |
| Possible target markets |
Description
The first step in proper cleaning of surgical instruments is to remove blood, body fluids, and tissues immediately after use. If cleaning is not performed
properly, the disinfection/sterilization steps will not be effective, leading to the exposure of patients to contaminants such as body fluids, tissues from other patients, and microorganisms.
The Chemdye® PRO 1 ENDO Hygiene Monitoring System is designed to verify the cleanliness of cannulated instruments by detecting protein residues that may remain after the cleaning process. The system is used in conjunction with Chemdye® SWE high absorption swabs, specially designed for cannulated instruments. These swabs must be inserted through the internal
channel of the cannulae to sample for contaminants that may remain after the cleaning process.
The Chemdye® PRO1 ENDO system provides quantitative results in the range of 1 to 50 μg of protein with a limit of detection (LOD) of 0.5 μg*, when incubated in the recommended Bionova® Auto-readers.
*Refers to µg BSA (bovine serum albumin).
Description
The first step in proper cleaning of surgical instruments is to remove blood, body fluids, and tissues immediately after use. If cleaning is not performed
properly, the disinfection/sterilization steps will not be effective, leading to the exposure of patients to contaminants such as body fluids, tissues from other patients, and microorganisms.
The Chemdye® PRO 1 ENDO Hygiene Monitoring System is designed to verify the cleanliness of cannulated instruments by detecting protein residues that may remain after the cleaning process. The system is used in conjunction with Chemdye® SWE high absorption swabs, specially designed for cannulated instruments. These swabs must be inserted through the internal
channel of the cannulae to sample for contaminants that may remain after the cleaning process.
The Chemdye® PRO1 ENDO system provides quantitative results in the range of 1 to 50 μg of protein with a limit of detection (LOD) of 0.5 μg*, when incubated in the recommended Bionova® Auto-readers.
*Refers to µg BSA (bovine serum albumin).
HAVE A QUESTION ABOUT OUR PRODUCTS?
WE´RE HERE TO HELP.