Test pack PCD for Steam (with one point integrator – purple)
Additional information
| Brand | |
|---|---|
| Process | Steam |
| Packaging | Single PCD |
| Regulations | ANSI/AAMI ST79, ISO 11138-3, ISO 11140-3 |
| Possible target markets |
Description
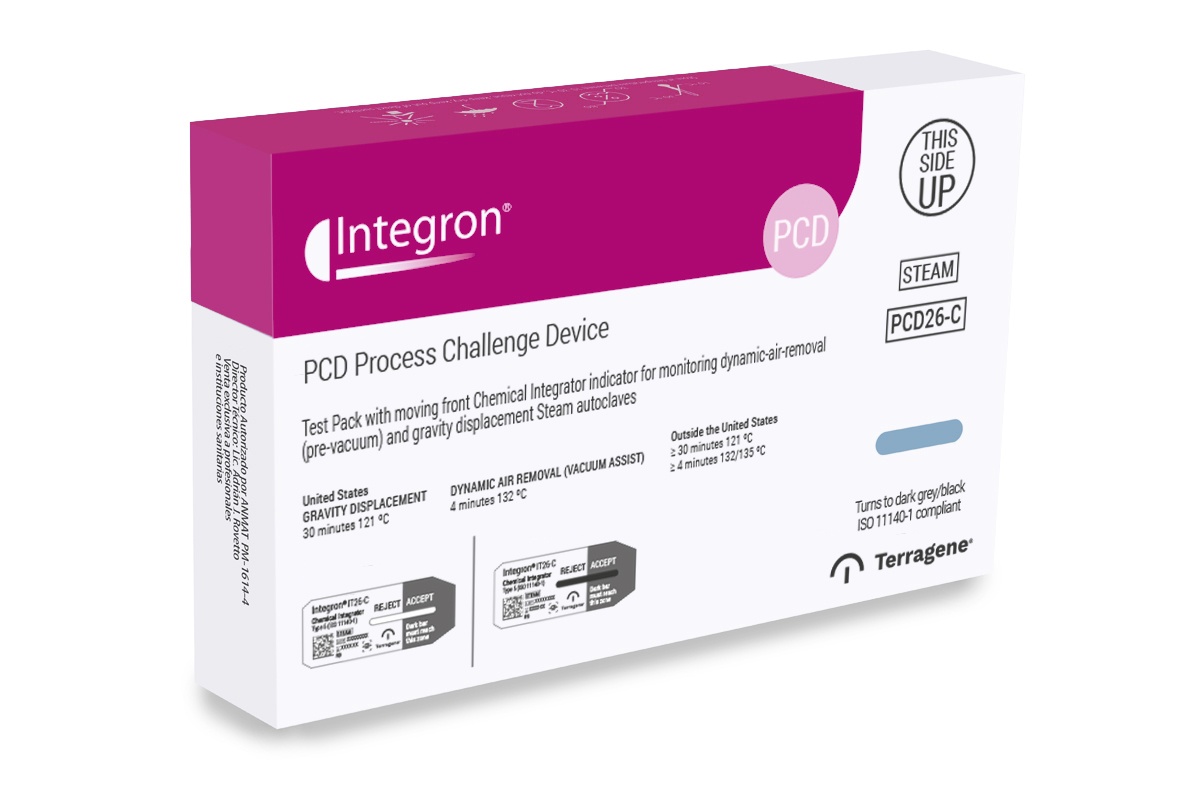
Process Challenge Device with self-adhesive Type 5 Chemical Indicator for monitoring autoclaves.
FDA cleared
Process Challenge Device Integron® PCD26-2 Test Pack was developed to detect inadequate air removal and steam penetration in dynamic-air removal (pre-vacuum) steam sterilizers at 132/135 °C 4 minutes and gravity displacement steam sterilizers at 121 °C 30 minutes.
The Test Pack consists of a porous barrier system equivalent to the challenge Test Pack specified in ANSI/AAMI ST79, holding in the middle a self-adhesive Record Card printed with Type 5 Integrator Indicator, where information about the sterilization cycle can be recorded. The indicator ink present on this self-adhesive card was developed to change to a reference color when a theoretical spore population reaches its time of death, indicating integration conditions were achieved.
This condition is calibrated with the kill time of a 106 Geobacillus stearothermophilus ATCC 7953 spore population, calculated in BIER (Biological Indicator Evaluator Resistometer). It also has a Type 1 Process Indicator located on the outside of the package, which allows to quickly identify if it has been exposed to the sterilization process, and to distinguish between processed and unprocessed units.
Please review the instructions for use for applicable cycles in your country
Instructions for Use
1- Place the pack inside a normally loaded steam autoclave, in those areas which are considered most inaccessible for the sterilizing agent (e.g., the center of the load and areas near the door).
2- Run the sterilization cycle.
3- After the sterilization process has finished, open the sterilizer door, wait for 5 minutes and remove the test pack. NOTE: The color of the box may vary from the original after undergoing the sterilization cycle. This does not represent a problem regarding the operation or quality of the product.
4- Check that the process indicator printed on box has changed color from light blue to grey. Open the test pack and remove the integrating indicator. PRECAUTION: Wear safety glasses and gloves when removing the indicator from the sterilized test pack.
5- Check the chemical integrator on the Record Card for correct exposure. Color change to the reference color confirms that the inside of the pack has been exposed to sufficient sterilization conditions. For chemical integrator reference color, please refer to Result Reference Guide. Otherwise, check the sterilization process.
6- Fill out the required information on the Record Card.
7- Discard the pack immediately
Description
Process Challenge Device with self-adhesive Type 5 Chemical Indicator for monitoring autoclaves.
FDA cleared
Process Challenge Device Integron® PCD26-2 Test Pack was developed to detect inadequate air removal and steam penetration in dynamic-air removal (pre-vacuum) steam sterilizers at 132/135 °C 4 minutes and gravity displacement steam sterilizers at 121 °C 30 minutes.
The Test Pack consists of a porous barrier system equivalent to the challenge Test Pack specified in ANSI/AAMI ST79, holding in the middle a self-adhesive Record Card printed with Type 5 Integrator Indicator, where information about the sterilization cycle can be recorded. The indicator ink present on this self-adhesive card was developed to change to a reference color when a theoretical spore population reaches its time of death, indicating integration conditions were achieved.
This condition is calibrated with the kill time of a 106 Geobacillus stearothermophilus ATCC 7953 spore population, calculated in BIER (Biological Indicator Evaluator Resistometer). It also has a Type 1 Process Indicator located on the outside of the package, which allows to quickly identify if it has been exposed to the sterilization process, and to distinguish between processed and unprocessed units.
Please review the instructions for use for applicable cycles in your country
Instructions for Use
1- Place the pack inside a normally loaded steam autoclave, in those areas which are considered most inaccessible for the sterilizing agent (e.g., the center of the load and areas near the door).
2- Run the sterilization cycle.
3- After the sterilization process has finished, open the sterilizer door, wait for 5 minutes and remove the test pack. NOTE: The color of the box may vary from the original after undergoing the sterilization cycle. This does not represent a problem regarding the operation or quality of the product.
4- Check that the process indicator printed on box has changed color from light blue to grey. Open the test pack and remove the integrating indicator. PRECAUTION: Wear safety glasses and gloves when removing the indicator from the sterilized test pack.
5- Check the chemical integrator on the Record Card for correct exposure. Color change to the reference color confirms that the inside of the pack has been exposed to sufficient sterilization conditions. For chemical integrator reference color, please refer to Result Reference Guide. Otherwise, check the sterilization process.
6- Fill out the required information on the Record Card.
7- Discard the pack immediately
HAVE A QUESTION ABOUT OUR PRODUCTS?
WE´RE HERE TO HELP.